Introduction: Motivation in Connection with Dozenism
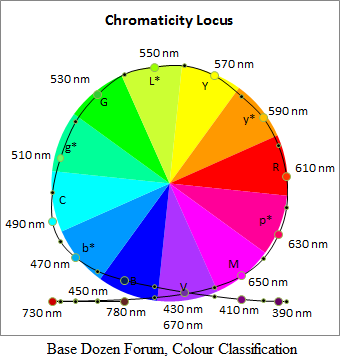
One application for base twelve where a division by three is thought to be convenient is in the classification of colour hues with reference to the three pigments of trichromatism. A representation of all the possible hues can be depicted in a planar cross-section through a three-dimensional space in which all colours can be plotted, for example by using as co-ordinates the amounts of three primary colours, most typically Red (R), Green (G), and Blue (B), forming the RGB colour space model used in description of colours produced by light-emitting diodes or phosphors in display screens of electrical devices. In the planar slice containing the hues, the three primary hues can be made to divide the full angle into three equal angles. For specifying these thirds of a unitarily or otherwise normalised full angle of turn conveniently by numerical fractions, a base containing the number three as a factor would be advantageous.
The Need for an Alternative System of Classification
Some have objected to the proposal of a Red-Green-Blue (RGB) co-ordinate system as a basis for naming colours because it is alleged to be not very intuitive in the connection between the colours produced and the rectangular co-ordinates and vice versa. However, it is possible to interconvert between different types of co-ordinate systems in three dimensions, such that other degrees of freedom for specifying colours can be used. For example, rectangular co-ordinates can be replaced by either cylindrical or spherical co-ordinates in a precise manner according to mathematical formulae. Variations on a cylindrical co-ordinate system theme for specification of colour include the so-called Hue-Saturation-Lightness (HSL) and Hue-Saturation-Value (HSV) classificatory systems. I disapprove of those systems in which all the fully saturated hues are in the same plane instead of being raised or lowered out of the plane by lightness or luma. The way in which the so-called Hue-Saturation-Lightness (HSL) model uses "lightness" is perceptually incorrect. I would prefer a spherical notation because of the globular shape of the visible gamut in accordance with personal experience and experimental elucidation such as by the Munsell system or by the International Commission on Illumination (CIE). A spherical co-ordinate system could impart the balanced advantages of greater conformance to perceptual regularity and a smaller proportion of unused or impossible colours in the space of the system according to the domains of available values for the co-ordinates.
Criticism of the Munsell System
The decimal steps in the Munsell system are abhorrent and could be transformed to dozenal increments. The Munsell so-called "Value" co-ordinate could be normalised to a range of zero to one, which could then be divided dozenally by simply writing the decimal numbers in dozenal format. The use of five principal hues in the Munsell system is an unnecessary complication, since all hue qualities can be specified by combinations of three hues in trichromatic vision, provided that the three primaries are chosen such that a neutral hueless grey or white devoid of chroma is circumscribed by them and that none of the primaries are complementary to each other. Additional primary hues can increase the gamut only in the sense of the extent of saturation. Another system of colour classification, the Pantone system, suffers from this defect of containing too many base colours. A further problem with the Munsell system is that it places too much emphasis on equal perceptual increments between the colours separated by the same numerical distance along its particular co-ordinates without taking into account physical characteristics that are objectively measurable experimentally using instruments rather than overreliance on perceptions of variable human observers. The ability of humans to discriminate colours at different wavelengths or hues varies from one individual to another depending on genetic differences between the colour receptors. Looking at the Munsell swatches, I see colours dumped into the same level that do not seem to have the same lightness and the variation along other dimensions does not always look consistent. I would say that better consistency is observable in models based on the colour cube of Red-Green-Blue (RGB). Basically, the Munsell system does not conform exactly regularly to how a human conceives of colour properties. It is inaccurate, though roughly approaching how colours should be classified in terms of their conceptual attributes. This is a reason for why the Munsell system is superseded in technological applications such as electronics by variants of systems devised in conjunction with the International Commission on Illumination (CIE), where the hues have been matched to wavelengths and lightness or luminance to some function of spectral radiance.
Criticism of the CIE systems
The experimental method from which the systems approved by the International Commission on Illumination (CIE) are derived involved somewhat arbitrary selection of hues at the particular wavelengths of 700 nanometres for "red", 546 nanometres for "green" and 436 nanometres for "blue", which are then called "standardised". It does not really matter exactly which wavelengths are used for the definition of the consequent space of colour hues, as long as the extreme ones are sufficiently far apart to cover the whole visible spectrum. Next, emissions at these wavelengths were combined to match the hues of monochromatic wavelengths. The results for the amounts of "red", "green", and "blue" as functions \(\bar{r}(\lambda), \bar{g}(\lambda), \bar{b}(\lambda)\) of the monochromatic wavelengths \(\lambda\) were then transformed to another rectangular co-ordinate system \(\bar{x}(\lambda), \bar{y}(\lambda), \bar{z}(\lambda)\) to avoid negative numbers and also in such a way that the middle function \(\bar{y}(\lambda)\), which has a peak at about 555 nanometres, would be the same as the photopic lightness or luminance. Perhaps with the realisation of there being a wavelength associated with a maximum of illuminance it would have been simpler to have used a monochromatic emitter with that wavelength as its peak for the middle primary hue. I interpret these functions as absorption spectra for wavelengths by hypothetical receptors which are not real. Any colour is then specified by a further set of rectangular co-ordinates \(X, Y, Z,\) called "tristimulus" values, which express how much that colour excites the respective hypothetical and unreal receptors. Actually, in my interpretation these co-ordinates are not really three separate stimuli, but represent combinations of stimuli. Thus for example, the luminance co-ordinate should be the result of a sum from a combination of excitation of all three receptors. For the expression of the colour space by chromaticity and luminance forming dimensions, yet another system of rectangular co-ordinates \(x, y, z\) was fabricated, which introduced an unnecessary shearing distortion transformation affecting the angle between the co-ordinate axes of the chromaticity reference plane. The result had a white point with rectangular co-ordinates at the third-of-the-way marks along the co-ordinate axes of the chromaticity diagram. In my opinion, the diagram would have been more useful for describing how a person conceives of colour in terms of qualities represented in co-ordinates if the white point had been at the origin of the chromaticity plane to which it is projected, though not necessarily the origin of the three-dimensional space. A white point at such an origin would have caused negative rectangular co-ordinates for the hues, but this would not negatively concern us if the hue is represented by a positive angular co-ordinate and the difference from white is represented by a positive radial co-ordinate as it appears when projected onto the chromaticity plane. In essence, the International Commission on Illumination (CIE) chromaticity co-ordinates are inept at representing human conceptions of colour qualities in the same way that the Red-Green-Blue system is unintuitive in its use of rectangular co-ordinates. Attempts have been made to produce cylindrical co-ordinate systems that are more perceptually uniform for specification of colours from the earlier International Commission on Illumination (CIE) rectangular systems.
(To be continued …)
References:
https://en.wikipedia.org/wiki/RGB_color_spaces
https://en.wikipedia.org/wiki/HSL_and_HSV
https://en.wikipedia.org/wiki/CIE_1931_color_space
https://en.wikipedia.org/wiki/Munsell_color_system
https://en.wikipedia.org/wiki/CIELAB_color_space#Cylindrical_model
https://en.wikipedia.org/wiki/CIELUV#Cylindrical_representation_(CIELCH)
One application for base twelve where a division by three is thought to be convenient is in the classification of colour hues with reference to the three pigments of trichromatism. A representation of all the possible hues can be depicted in a planar cross-section through a three-dimensional space in which all colours can be plotted, for example by using as co-ordinates the amounts of three primary colours, most typically Red (R), Green (G), and Blue (B), forming the RGB colour space model used in description of colours produced by light-emitting diodes or phosphors in display screens of electrical devices. In the planar slice containing the hues, the three primary hues can be made to divide the full angle into three equal angles. For specifying these thirds of a unitarily or otherwise normalised full angle of turn conveniently by numerical fractions, a base containing the number three as a factor would be advantageous.
The Need for an Alternative System of Classification
Some have objected to the proposal of a Red-Green-Blue (RGB) co-ordinate system as a basis for naming colours because it is alleged to be not very intuitive in the connection between the colours produced and the rectangular co-ordinates and vice versa. However, it is possible to interconvert between different types of co-ordinate systems in three dimensions, such that other degrees of freedom for specifying colours can be used. For example, rectangular co-ordinates can be replaced by either cylindrical or spherical co-ordinates in a precise manner according to mathematical formulae. Variations on a cylindrical co-ordinate system theme for specification of colour include the so-called Hue-Saturation-Lightness (HSL) and Hue-Saturation-Value (HSV) classificatory systems. I disapprove of those systems in which all the fully saturated hues are in the same plane instead of being raised or lowered out of the plane by lightness or luma. The way in which the so-called Hue-Saturation-Lightness (HSL) model uses "lightness" is perceptually incorrect. I would prefer a spherical notation because of the globular shape of the visible gamut in accordance with personal experience and experimental elucidation such as by the Munsell system or by the International Commission on Illumination (CIE). A spherical co-ordinate system could impart the balanced advantages of greater conformance to perceptual regularity and a smaller proportion of unused or impossible colours in the space of the system according to the domains of available values for the co-ordinates.
Criticism of the Munsell System
The decimal steps in the Munsell system are abhorrent and could be transformed to dozenal increments. The Munsell so-called "Value" co-ordinate could be normalised to a range of zero to one, which could then be divided dozenally by simply writing the decimal numbers in dozenal format. The use of five principal hues in the Munsell system is an unnecessary complication, since all hue qualities can be specified by combinations of three hues in trichromatic vision, provided that the three primaries are chosen such that a neutral hueless grey or white devoid of chroma is circumscribed by them and that none of the primaries are complementary to each other. Additional primary hues can increase the gamut only in the sense of the extent of saturation. Another system of colour classification, the Pantone system, suffers from this defect of containing too many base colours. A further problem with the Munsell system is that it places too much emphasis on equal perceptual increments between the colours separated by the same numerical distance along its particular co-ordinates without taking into account physical characteristics that are objectively measurable experimentally using instruments rather than overreliance on perceptions of variable human observers. The ability of humans to discriminate colours at different wavelengths or hues varies from one individual to another depending on genetic differences between the colour receptors. Looking at the Munsell swatches, I see colours dumped into the same level that do not seem to have the same lightness and the variation along other dimensions does not always look consistent. I would say that better consistency is observable in models based on the colour cube of Red-Green-Blue (RGB). Basically, the Munsell system does not conform exactly regularly to how a human conceives of colour properties. It is inaccurate, though roughly approaching how colours should be classified in terms of their conceptual attributes. This is a reason for why the Munsell system is superseded in technological applications such as electronics by variants of systems devised in conjunction with the International Commission on Illumination (CIE), where the hues have been matched to wavelengths and lightness or luminance to some function of spectral radiance.
Criticism of the CIE systems
The experimental method from which the systems approved by the International Commission on Illumination (CIE) are derived involved somewhat arbitrary selection of hues at the particular wavelengths of 700 nanometres for "red", 546 nanometres for "green" and 436 nanometres for "blue", which are then called "standardised". It does not really matter exactly which wavelengths are used for the definition of the consequent space of colour hues, as long as the extreme ones are sufficiently far apart to cover the whole visible spectrum. Next, emissions at these wavelengths were combined to match the hues of monochromatic wavelengths. The results for the amounts of "red", "green", and "blue" as functions \(\bar{r}(\lambda), \bar{g}(\lambda), \bar{b}(\lambda)\) of the monochromatic wavelengths \(\lambda\) were then transformed to another rectangular co-ordinate system \(\bar{x}(\lambda), \bar{y}(\lambda), \bar{z}(\lambda)\) to avoid negative numbers and also in such a way that the middle function \(\bar{y}(\lambda)\), which has a peak at about 555 nanometres, would be the same as the photopic lightness or luminance. Perhaps with the realisation of there being a wavelength associated with a maximum of illuminance it would have been simpler to have used a monochromatic emitter with that wavelength as its peak for the middle primary hue. I interpret these functions as absorption spectra for wavelengths by hypothetical receptors which are not real. Any colour is then specified by a further set of rectangular co-ordinates \(X, Y, Z,\) called "tristimulus" values, which express how much that colour excites the respective hypothetical and unreal receptors. Actually, in my interpretation these co-ordinates are not really three separate stimuli, but represent combinations of stimuli. Thus for example, the luminance co-ordinate should be the result of a sum from a combination of excitation of all three receptors. For the expression of the colour space by chromaticity and luminance forming dimensions, yet another system of rectangular co-ordinates \(x, y, z\) was fabricated, which introduced an unnecessary shearing distortion transformation affecting the angle between the co-ordinate axes of the chromaticity reference plane. The result had a white point with rectangular co-ordinates at the third-of-the-way marks along the co-ordinate axes of the chromaticity diagram. In my opinion, the diagram would have been more useful for describing how a person conceives of colour in terms of qualities represented in co-ordinates if the white point had been at the origin of the chromaticity plane to which it is projected, though not necessarily the origin of the three-dimensional space. A white point at such an origin would have caused negative rectangular co-ordinates for the hues, but this would not negatively concern us if the hue is represented by a positive angular co-ordinate and the difference from white is represented by a positive radial co-ordinate as it appears when projected onto the chromaticity plane. In essence, the International Commission on Illumination (CIE) chromaticity co-ordinates are inept at representing human conceptions of colour qualities in the same way that the Red-Green-Blue system is unintuitive in its use of rectangular co-ordinates. Attempts have been made to produce cylindrical co-ordinate systems that are more perceptually uniform for specification of colours from the earlier International Commission on Illumination (CIE) rectangular systems.
(To be continued …)
References:
https://en.wikipedia.org/wiki/RGB_color_spaces
https://en.wikipedia.org/wiki/HSL_and_HSV
https://en.wikipedia.org/wiki/CIE_1931_color_space
https://en.wikipedia.org/wiki/Munsell_color_system
https://en.wikipedia.org/wiki/CIELAB_color_space#Cylindrical_model
https://en.wikipedia.org/wiki/CIELUV#Cylindrical_representation_(CIELCH)











» Dozenal Number Words from Metric Prefixes
» Dozenalizing Metric
» Myon Dozenal Nomenclature
» Information per Area of Numerical Forms
» Denominational Dozenal Numerals
» Proto-Indo-European Numbers
» Radix Economy for Alternating Bases
» Graduation Subdivisions